A New Option: COVID-19 Testing at Home
You can now take a test for COVID-19 positivity in the comfort and safety of your home.
Samples for your test-by-mail kits should be shipped back by you on the same day that you collected the sample, in order to minimize the transit time between your home and the testing lab.
On November 17th, 2020, the FDA authorized the first COVID-19 diagnostic test to self-test at home: the Lucira COVID-19 All-In-One Test Kit. Since then, the FDA has approved dozens of self-diagnostic tests.
There are currently two varieties: test-by-mail kits, in which users mail samples to a laboratory, and at-home tests, which give a result right away. Both options require users to purchase a kit costing from $25 to over $100.
For test-by-mail kits, such as the Pixel COVID-19 Test, users take an in vitro sample and ship it to a laboratory for examination. On average, these laboratories need three to five days to process the sample and convey the result to the user since they use a method called polymerase chain reaction (PCR) in order to detect the Coronavirus.
PCR works by amplifying specific gene sequences in order to detect the virus. These tests can identify even the smallest amount of virus and have an extremely high accuracy rate. Even though a negative result from the PCR test is not necessarily a true negative, it gives a high level of assurance to the user.
The at-home tests are carried out by swabbing both nostrils and bathing the swab in a liquid. The wait time for the results depends on the brand used. For example, the Lucira test kit is designed to detect positive results in as little as eleven minutes and confirm negative results in thirty minutes.
Many of the at-home tests, such as those made by Ellume and Abbott, are antigen tests that look for antibodies to the Coronavirus, which can help identify the individuals who have developed an immune response to the virus. Because they are designed for rapid detection of the virus, antigen tests may not detect all active infections, and accuracy in asymptomatic users is less than 50 percent.
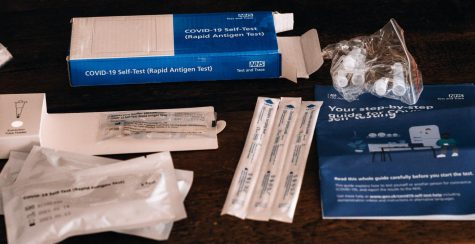
False negatives occur due to technical errors where the user collects a specimen incorrectly, or in cases when the test was taken too early, prior to the individual’s infection with COVID-19. During the early stages of the Coronavirus infection, the body will have a small amount of virus which can be hard to detect. Therefore, waiting a few days or taking the test-by-mail through PCR is best to ensure that the user to safe to visit friends. But if you are in a hurry, the at-home test will be the best choice.
The cost of at-home and test-by-mail tests varies from brand to brand. At-home tests are relatively cheap, costing from $25 to $50, while test-by-mail kits are over $100 and may not be reimbursed by health insurance companies. It is the user’s responsibility to contact their insurance carrier to discuss reimbursement, which must be deemed medically appropriate by a healthcare provider. Fortunately, the Pixel test kit allows individuals to not pay an upfront cost when they meet one or more of the clinical guidelines for COVID-19 testing.
Hopefully, as the market for at-home testing for COVID-19 expands, there will be more options and prices will become cheaper. The more affordable they are, the more encouraged consumers are to buy the kits for routine testing. Especially now with the reopening of public facilities, testing is as crucial as ever to mitigate the spread of the Coronavirus, along with widespread vaccination.
On November 17th, 2020, the FDA authorized the first COVID-19 diagnostic test to self-test at home: the Lucira COVID-19 All-In-One Test Kit. Since then, the FDA has approved dozens of self-diagnostic tests.
Nicole Hu is a Staff Reporter for 'The Science Survey.' She enjoys reading articles about new discoveries and the unknown. They are informative and allow...

